Brownian motion
All matter is made of tiny particles.
The particles are atoms, ions or molecules.
In a liquid or gas, the particles move at random.
If any small enough substance is placed in between these particles it will also be in a random motion known as Brownian motion.
This motion happens in liquids and gases as their particles move around it randomly and collide with each other , i.e it is being bombarded by the other moving particles.
Even larger particles can be moved by light-weight fast moving particles.
this is an evidence for the kinetic particle model of matter as it shows that matter is made of individual particles.
Scientist Robert Brown observed in the year 1827 by using a microscope rapid random movement of pollen grains within wate, he could not explain the reason but later Enistein showed that there are separate particles in the water.
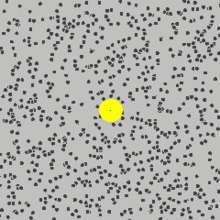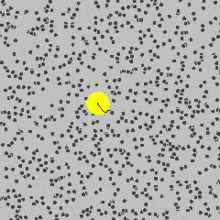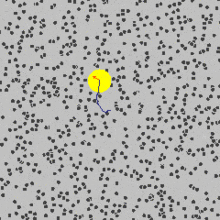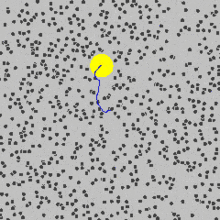
that are moving randomly and caused pollen grains to move.You can see in the picture above how the yellow sphere is moving due to being bombarded by the fast random moving particles.
So now you can define
Brownian motion
as
Brownian motion is the random movement of particles in a fluid due to their collisions with other atoms or molecules.
Download as PDF_ :
what is BROWNIAN motion ?

No comments:
Post a Comment